Real-time insights for research and clinical trials
Experience Sampling • EMA • eCOA • ePRO • eDiary
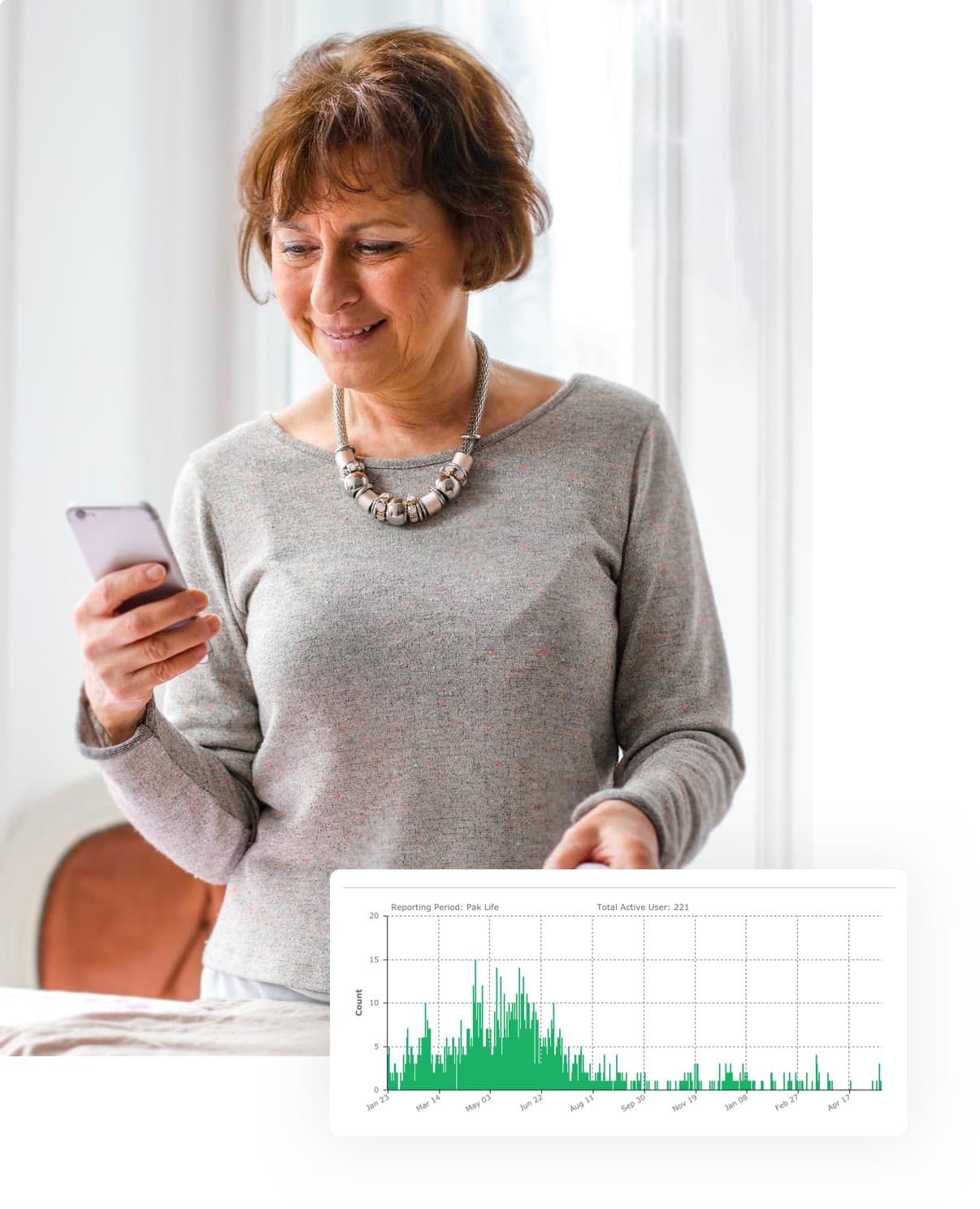
Streamlined tools to enroll, monitor, and support participants without spreadsheets.
Advanced display logic, piped text, scores, and schedules to auto-adapt the study experience to each participant.
Smart reminders and response tracking help maximize compliance, while alerts let you step in at the right time.
Well-labeled datasets in multiple formats with timestamps, scores, and session metadata; ready for immediate analysis.
Default dashboards and templates reveal engagement and responses in real time with flexible customization for your study.
Native iOS and Android apps built for efficient, secure, and user-friendly data collection; online or offline.
LifeData in numbers
How it works
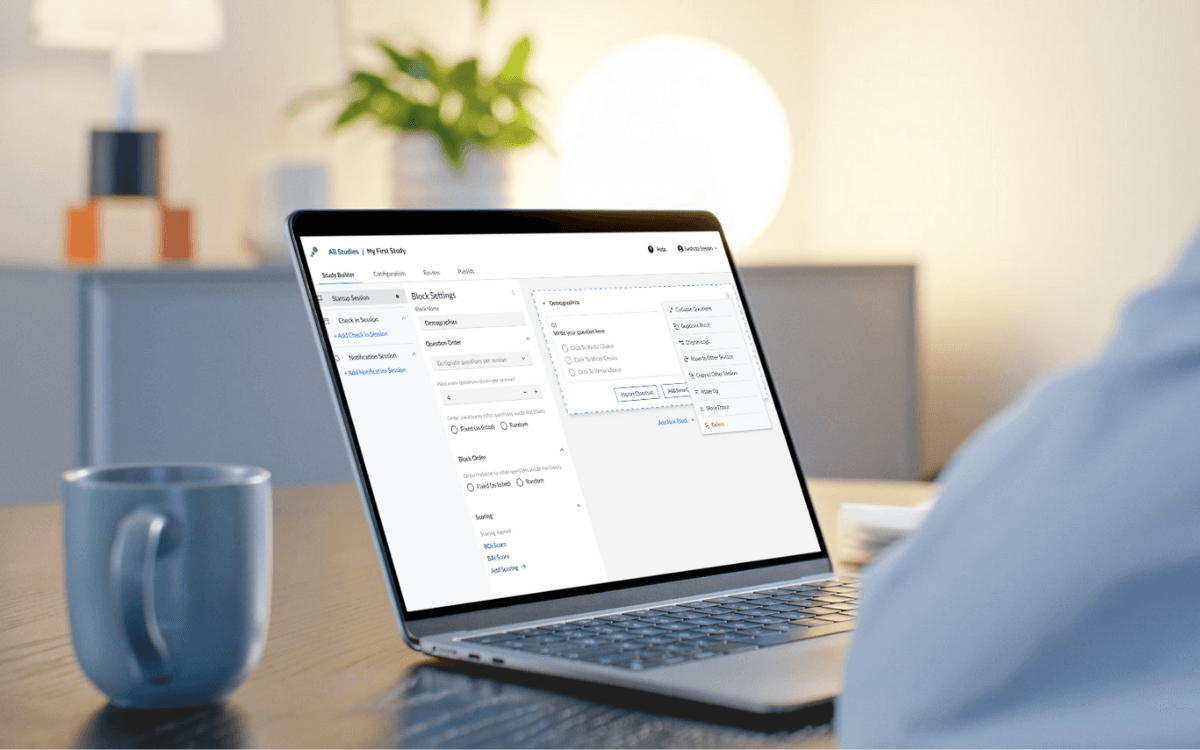
Design Your Study
Build your study by creating questions and schedules in one place. Everything is packaged as a single complete study that’s ready to launch.
Onboard Participants
Participants can join in under a minute using a simple email link or QR code.

Access Your Data
Access your data anytime. Export clean .csv files or explore real-time dashboards for instant insights
Feature highlights
Experience Sampling
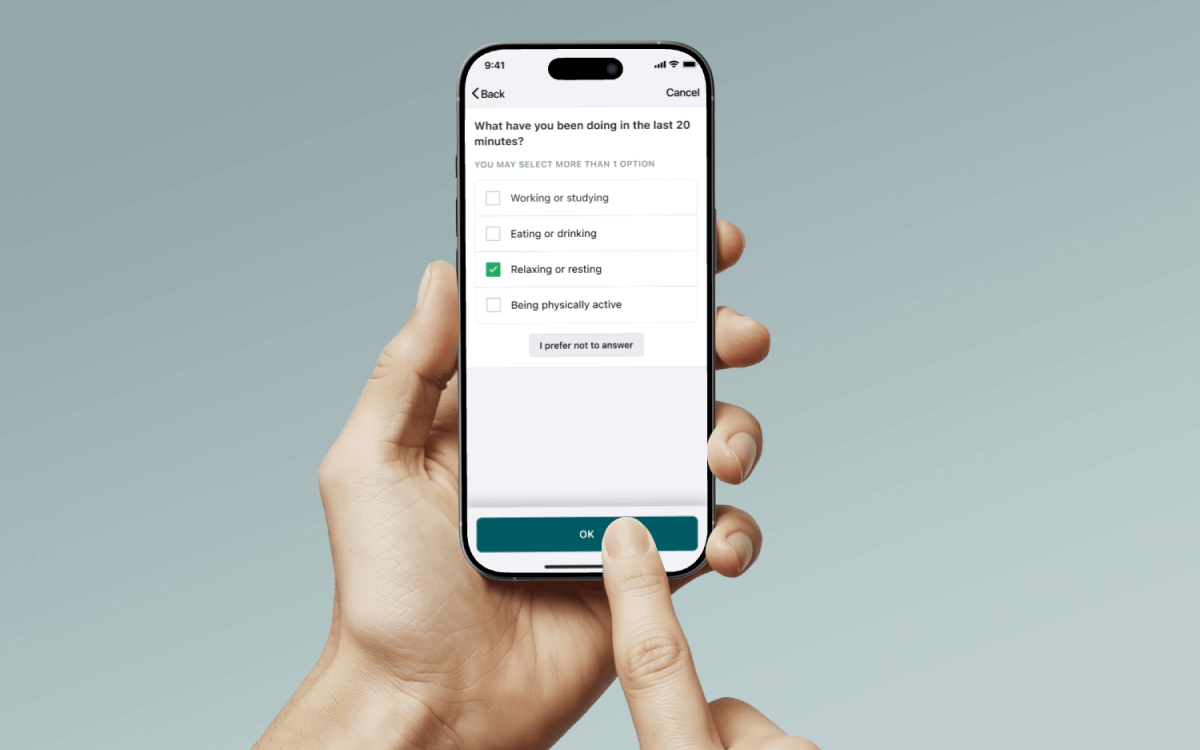
Deliver in the moment questions via notification. Participants tap the notification allowing them to answer questions right away. Flexible scheduling options and the ability to create multiple question/schedule combinations accommodate even the most complex study designs.
eDiary
Participants can respond to questions in the mobile app on their own time. This feature is useful for event-based reporting, daily diary studies, or providing always available intervention materials.
Flexible notification scheduling options allow you to tailor data gathering to fit your study design. Questions can be scheduled for delivery at specific (fixed) times or at random times within specified time windows.
Fixed Example: Send questions at 8 am, 12pm, and 5pm to study mealtimes.
Random Example: Send questions at three random times between 8am and 5pm, at least 90 minutes apart, to assess mood fluctuation throughout the workday.
Immediate Follow-Up: “Branching”
Use branching to ask immediate follow-up questions based off how your participants respond.
Delayed Follow-Up: “Triggering”
Use participants’ responses to trigger a later notification and follow-up questions. There are two types of triggers. One type sends questions at a later point in time (e.g., 90 minutes later), and the other type of trigger activates a whole new schedule of questions (e.g., once per day for a week).
Once participants load the RealLife Exp app and study protocol onto their phones, all data collection can be handled offline. This allows data gathering to go on reliably, regardless of whether participants have connectivity problems. When participants connect to WiFi or have a cellular signal, their responses are automatically uploaded to the server.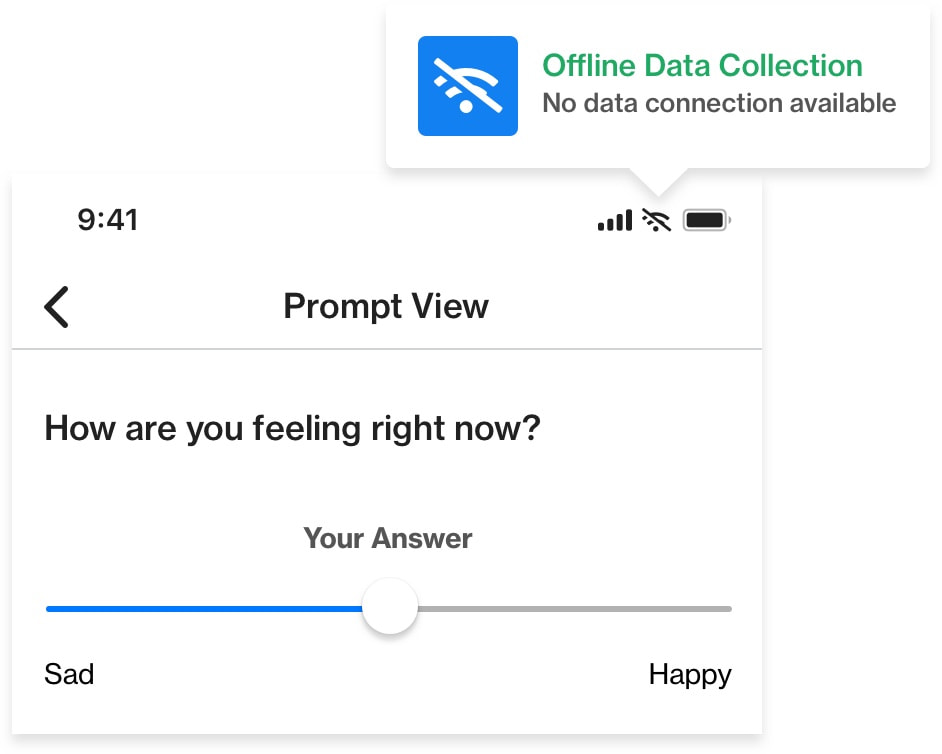
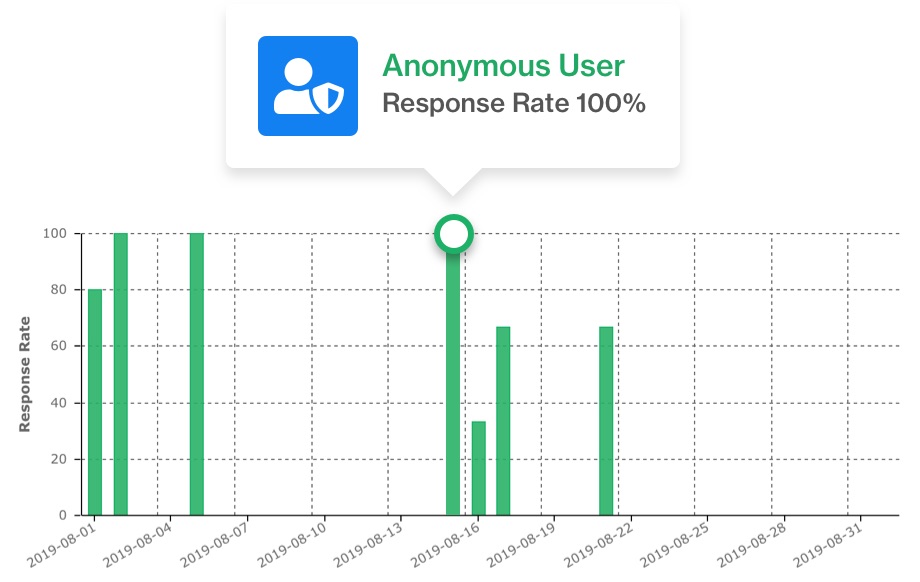
Participant data can be gathered using an anonymous mode which allows them to engage with the mobile app without providing personally identifiable information.
Participants can easily download our RealLife Exp mobile app to their phone using rapid onboarding via an email link or QR code, or by App Store Search. Want to see how rapid onboarding works? Tap this link from your mobile phone, or scan the QR Code below to load the app and a demo study protocol.
Work with team members on projects and assign roles with varying levels of system and data access.

Easy HIPAA/GDPR compliance
- Gather data without Personally Identifiable Information (PII)
- Comply with IRB/HIPAA requirements
- Comply with the Data Privacy Framework for the European Union, United Kingdom, and Switzerland

Hundreds of institutions in 65 countries trust LifeData
Used in studies published in top journals